| NCT03742102 | Triple Negative Breast Neoplas... more >>ms Collapse << | Phase 1
Phase 2 | Not yet recruiting | June 3, 2022 | United States, Arizona
... more >>
Research Site
Not yet recruiting
Goodyear, Arizona, United States, 85338
Research Site
Not yet recruiting
Tucson, Arizona, United States, 85712
United States, California
Research Site
Not yet recruiting
Los Angeles, California, United States, 90048
United States, Florida
Research Site
Not yet recruiting
New Port Richey, Florida, United States, 34653
United States, Georgia
Research Site
Not yet recruiting
Newnan, Georgia, United States, 30265
United States, Illinois
Research Site
Not yet recruiting
Zion, Illinois, United States, 60099
United States, Missouri
Research Site
Not yet recruiting
Saint Louis, Missouri, United States, 63110
United States, Oklahoma
Research Site
Not yet recruiting
Tulsa, Oklahoma, United States, 74133
United States, Pennsylvania
Research Site
Not yet recruiting
Philadelphia, Pennsylvania, United States, 19124
United States, Utah
Research Site
Not yet recruiting
Salt Lake City, Utah, United States, 84112
Korea, Republic of
Research Site
Not yet recruiting
Seoul, Korea, Republic of, 03080
Research Site
Not yet recruiting
Seoul, Korea, Republic of, 05505
Research Site
Not yet recruiting
Seoul, Korea, Republic of, 06351
Taiwan
Research Site
Not yet recruiting
Taichung, Taiwan, 40447
Research Site
Not yet recruiting
Taipei, Taiwan, 100
United Kingdom
Research Site
Not yet recruiting
London, United Kingdom, EC1M 6BQ
Research Site
Not yet recruiting
London, United Kingdom, W1T 7HA
Research Site
Not yet recruiting
Manchester, United Kingdom
Research Site
Not yet recruiting
Oxford, United Kingdom, OX3 7LE
Research Site
Not yet recruiting
Truro, United Kingdom, TR1 3LJ Collapse << |
| NCT02465060 | Advanced Malignant Solid Neopl... more >>asm
Bladder Carcinoma
Breast Carcinoma
Cervical Carcinoma
Colon Carcinoma
Colorectal Carcinoma
Endometrial Carcinoma
Esophageal Carcinoma
Gastric Carcinoma
Glioma
Head and Neck Carcinoma
Kidney Carcinoma
Liver and Intrahepatic Bile Duct Carcinoma
Lung Carcinoma
Lymphoma
Malignant Uterine Neoplasm
Melanoma
Ovarian Carcinoma
Pancreatic Carcinoma
Plasma Cell Myeloma
Prostate Carcinoma
Rectal Carcinoma
Recurrent Bladder Carcinoma
Recurrent Breast Carcinoma
Recurrent Cervical Carcinoma
Recurrent Colon Carcinoma
Recurrent Colorectal Carcinoma
Recurrent Esophageal Carcinoma
Recurrent Gastric Carcinoma
Recurrent Glioma
Recurrent Head and Neck Carcinoma
Recurrent Liver Carcinoma
Recurrent Lung Carcinoma
Recurrent Lymphoma
Recurrent Malignant Solid Neoplasm
Recurrent Melanoma
Recurrent Ovarian Carcinoma
Recurrent Pancreatic Carcinoma
Recurrent Plasma Cell Myeloma
Recurrent Prostate Carcinoma
Recurrent Rectal Carcinoma
Recurrent Skin Carcinoma
Recurrent Thyroid Gland Carcinoma
Recurrent Uterine Corpus Carcinoma
Refractory Lymphoma
Refractory Malignant Solid Neoplasm
Refractory Plasma Cell Myeloma
Skin Carcinoma
Thyroid Gland Carcinoma
Uterine Corpus Cancer Collapse << | Phase 2 | Recruiting | - | - |
| NCT02664935 | Non-Small Cell Lung Cancer
... more >> Carcinoma, Squamous Cell
Adenocarcinoma Collapse << | Phase 2 | Recruiting | September 2021 | United Kingdom
... more >> Belfast City Hospital, Belfast Health and Social Care Trust
Recruiting
Belfast, United Kingdom
Contact: Paula Scullin
Queen Elizabeth Hospital Birmingham, University Hospitals Birmingham NHS Foundation Trust
Recruiting
Birmingham, United Kingdom, B15 2GW
Contact: Gary Middleton, MB BS MD FRCP
Principal Investigator: Gary Middleton, MB BS MD FRCP
Birmingham Heartlands Hospital, Heart of England NHS Foundation Trust
Recruiting
Birmingham, United Kingdom
Addenbrooke's Hospital, Cambridge University Hospitals NHS Foundation Trust
Recruiting
Cambridge, United Kingdom, CB2 0QQ
Contact: David Gilligan, BSc (Hons) MB BChir FRCP FRCR cctc@addenbrookes.nhs.uk
Principal Investigator: David Gilligan, BSc (Hons) MB BChir FRCP FRCR
Velindre Cancer Centre, Velindre NHS Trust
Recruiting
Cardiff, United Kingdom, CF14 2TL
Contact: Alison Brewster, BSC MB BCh FRCR MD 029 2061 5888
Principal Investigator: Alison Brewster, BSC MB BCh FRCR MD
Edinburgh Cancer Centre, Western General Hospital
Recruiting
Edinburgh, United Kingdom, EH4 2XU
Contact: Melanie Mackean, MB ChB MRCP MSc MD
Principal Investigator: Melanie Mackean, MB ChB MRCP MSc MD
Royal Devon and Exeter Hospital
Recruiting
Exeter, United Kingdom
Contact: Elizabeth Toy
Principal Investigator: Elizabeth Toy
Cancer Research UK Clinical Trials Unit, Beatson West of Scotland Cancer Centre
Recruiting
Glasgow, United Kingdom, G12 0YN
Contact: Noelle O'Rourke, BA MB MCh MA MCRP MD FRCR CCST
Principal Investigator: Noelle O'Rourke, BA MB MCh MA MCRP MD FRCR CCST
St. James' University Hospital, Leeds Teaching Hospital NHS Trust
Recruiting
Leeds, United Kingdom, LS9 7TF
Contact: Clive Mulatero, BA MB BCh MA PhD FRCP
Principal Investigator: Clive Mulatero, BA MB BCh MA PhD FRCP
Leicester Royal Infirmary, University Hospitals of Leicester NHS Trust
Recruiting
Leicester, United Kingdom
Contact: Dean Fennell
Royal Marsden Hospital, The Royal Marsden NHS Foundation Trust
Recruiting
London, United Kingdom, SW3 6JJ
Contact: Sanjay Popat
Principal Investigator: Sanjay Popat, BSc MB BS MRCP PhD FRCP
Charing Cross Hospital, Imperial College Healthcare NHS Trust
Recruiting
London, United Kingdom
Contact: Conrad Lewanski
Guy's Hospital, Guy's and St Thomas' NHS Foundation Trust
Recruiting
London, United Kingdom
Contact: James Spicer
St Bartholomew's Hospital, Barts Health NHS Trust
Recruiting
London, United Kingdom
Contact: John Conibear
University College Hospital, University College London Hospitals NHS Foundation Trust
Recruiting
London, United Kingdom
The Christie Hospital, The Christie NHS Foundation Trust
Recruiting
Manchester, United Kingdom
Contact: Yvonne Summers
Sir Bobby Robson Cancer Trial Research Centre, The Newcastle upon Tyne Hospitals
Recruiting
Newcastle, United Kingdom, NE7 7DN
Contact: Alastair Greystoke
Principal Investigator: Alastair Greystoke, BSc MBChB MRCP MSc PhD
Churchill Hospital, Oxford University Hospitals NHS Foundation Trust
Recruiting
Oxford, United Kingdom
Contact: Denis Talbot
Weston Park Hospital, Sheffield Teaching Hospitals NHS Foundation Trust
Recruiting
Sheffield, United Kingdom
Contact: Sarah Danson
Southampton General Hospital, University Hospital Southampton NHS Foundation Trust
Recruiting
Southampton, United Kingdom, SO16 6YD
Contact: Christian Ottensmeier clinicaltrials@uhs.nhs.uk
Principal Investigator: Christian Ottensmeier Collapse << |
| NCT02117167 | Non-small Cell Lung Cancer Met... more >>astatic Collapse << | Phase 2 | Recruiting | February 2022 | France
... more >> Centre Hospitalier Henri Duffau
Recruiting
Avignon, France
Contact: Nicolas CLOAREC, MD nicolas.cloarec@ch-avignon.fr
Principal Investigator: Nicolas CLOAREC, MD
Centre Hospitalier Universitaire de Besancon - Hopital Jean Minjoz
Recruiting
Besancon, France
Contact: Virginie WESTEEL, MD virginie.westeel@univ.fcomte.fr
Principal Investigator: Virginie WESTEEL, MD
Hôpital Avicenne
Recruiting
Bobigny, France
Contact: Boris DUCHEMANN, MD boris.duchemann@aphp.fr
Principal Investigator: Boris DUCHEMANN, MD
Institut Bergonié
Recruiting
Bordeaux, France
Contact: François Chomy, MD f.chomy@bordeaux.unicancer.fr
Principal Investigator: François Chomy, MD
Hôpital Ambroise Paré
Recruiting
Boulogne Billancourt, France
Contact: Etienne GIROUX LEPRIEUR, MD etienne.giroux-leprieur@aphp.fr
Principal Investigator: Etienne GIROUX LEPRIEUR, MD
Hospices Civils de Lyon- Hôpital Louis Pradel
Recruiting
Bron, France
Contact: Nicolas Girard, MD nicolas.girard@chu-lyon.fr
Principal Investigator: Nicolas Girard, MD
Centre François Baclesse
Recruiting
Caen, France
Contact: Radj Gervais, MD r.gervais@baclesse.unicancer.fr
Principal Investigator: Radj Gervais, MD
CHU Caen
Recruiting
Caen, France
Contact: Gérard Zalcman, MD zalcman-g@chu-caen.fr
Principal Investigator: Gérard Zalcman, MD
Chu de Caen - Hopital Cote de Nacre
Recruiting
Caen, France
Contact: Jeannick MADELAINE, MD madelaine-j@chu-caen.fr
Principal Investigator: Jeannick MADELAINE, MD
Hôpital Louis Pasteur
Recruiting
Chartres, France
Contact: Claire Lethrosne, MD clethrosne@ch-chartres.fr
Principal Investigator: Claire Lethrosne, MD
centre Jean Perrin
Recruiting
Clermont-Ferrand, France
Contact: Xavier Durando, MD xavier.durando@cjp.fr
Principal Investigator: Xavier Durando, MD
CHU Clermont Ferrand - Hôpital Gabriel Montpied
Recruiting
Clermont-Ferrand, France
Contact: Henri Janicot, MD hjanicot@chu-clermontferrand.fr
Principal Investigator: Henri Janicot, MD
Hopitaux Civils de Colmar
Recruiting
Colmar, France
Contact: Lionel MOREAU, MD lionel.moreau@ch-colmar.fr
Principal Investigator: Lionel MOREAU, MD
Centre Hopsitalier Intercommunal de Créteil
Recruiting
Créteil, France
Contact: Isabelle Monnet, MD isabelle.monnet@chicreteil.fr
Principal Investigator: Isabelle Monnet, MD
Centre Georges François Leclerc
Recruiting
Dijon, France
Contact: Bruno Coudert, MD bcoudert@cgfl.fr
Principal Investigator: Bruno Coudert, MD
CHU Grenoble
Recruiting
Grenoble, France
Contact: Denis Moro-Sibilot, MD Dmoro-Sibilot@chu-grenoble.fr
Principal Investigator: Denis Moro-Sibilot, MD
Chd Vendee
Recruiting
La Roche Sur Yon, France
Contact: Tifenn L'HARIDON, MD
Principal Investigator: Tifenn L'HARIDON, MD
CH du Mans
Recruiting
Le Mans, France
Contact: Olivier Molinier, MD omolinier@ch-lemans.fr
Principal Investigator: Olivier Molinier, MD
Centre Oscar Lambret
Recruiting
Lille, France
Contact: Eric Dansin, MD e-dansin@o-lambret.fr
Principal Investigator: Eric Dansin, MD
CHRU de Lille
Recruiting
Lille, France
Contact: Alexis Cortot, MD Alexis.CORTOT@CHRU-LILLE.FR
Principal Investigator: Alexis Cortot, MD
Centre Léon Bérard
Recruiting
Lyon, France
Contact: Maurice Pérol, MD maurice.perol@lyon.unicancer.fr
Principal Investigator: Maurice Pérol, MD
Hôpital Nord
Recruiting
Marseille, France
Contact: Fabrice Barlesi, MD fabrice.barlesi@ap-hm.fr
Principal Investigator: Fabrice Barlési, MD
Institut Paoli Calmettes
Recruiting
Marseille, France
Contact: Anne MADROSZYK, MD madroszyka@ipc.unicancer.fr
Principal Investigator: Anne MADROSZYK, MD
Institut de cancérologie de l'Ouest
Recruiting
Nantes, France
Contact: Jaafar Bennouna, MD jaafar.bennouna@ico.unicancer.fr
Principal Investigator: Jaafar Bennouna, MD
Centre Antoine Lacassagne
Recruiting
Nice, France
Contact: Josiane OTTO, MD josiane.otto@nice.unicancer.fr
Principal Investigator: Josiane OTTO, MD
Chr Orleans
Recruiting
Orleans, France
Contact: Hugues MOREL, MD hugues.morel@chr-orleans.fr
Contact: , MD
Principal Investigator: Hugues MOREL
AH-HP Hôpital Saint Louis
Recruiting
Paris, France
Contact: Damien Pouessel, MD damien.pouessel@sls.aphp.fr
Principal Investigator: Damien Pouessel, MD
AP-HP Hôpital Cochin
Recruiting
Paris, France
Contact: Jeanne Chapron, MD jeanne.chapron@cch.aphp.fr
Principal Investigator: Jeanne Chapron, MD
AP-HP Hôpital Européen Georges Pompidou
Withdrawn
Paris, France
AP-HP Hôpital Tenon
Recruiting
Paris, France
Contact: Marie Wislez, MD marie.wislez@tnn.aphp.fr
Principal Investigator: Marie Wislez, MD
Institut Curie
Recruiting
Paris, France
Contact: Catherine Daniel, MD catherine.daniel@curie.fr
Principal Investigator: Catherine Daniel, MD
Centre Hospitalier de Pau
Recruiting
PAU, France
Contact: Aldo RENAULT, MD aldo.renault@ch-pau.fr
Principal Investigator: Aldo RENAULT, MD
Centre Hospitalier Lyon Sud
Recruiting
Pierre Bénite, France
Contact: Jean-Pierre Souquet, MD pierre-jean.souquet@chu-lyon.fr
Principal Investigator: Jean-Pierre Souquet, MD
CHR Pontchailloux
Withdrawn
Rennes, France
Chru Strasbourg - Nouvel Hopital Civil
Recruiting
Strasbourg, France
Contact: Philippe BARTHELEMY, MD
Principal Investigator: Philippe BARTHELEMY, MD
CHI de Toulon - Hôpital Sainte-Musse
Recruiting
Toulon, France
Contact: Xavier TCHIKNAVORIAN, MD
Principal Investigator: Xavier TCHIKNAVORIAN, MD
CHU Toulouse -Hôpital Larrey
Recruiting
Toulouse, France
Contact: Julien Mazières, MD mazieres.j@chu-toulouse.fr
Principal Investigator: Julien Mazières, MD
Hôpital Bretonneau
Recruiting
Tours, France
Contact: Eric Pichon, MD e.pichon@chu-tours.fr
Principal Investigator: Eric Pichon, MD
Gustave Roussy
Recruiting
Villejuif, France
Contact: Benjamin Besse, MD Benjamin.BESSE@gustaveroussy.fr
Principal Investigator: Benjamin Besse, MD
Sub-Investigator: Jean-Charles Soria, MD Collapse << |
| NCT02299999 | Metastatic Breast Cancer | Phase 2 | Recruiting | December 2022 | France
... more >> Institut de Cancérologie de l'Ouest/Paul Papin
Recruiting
Angers, France
Contact: Mario CAMPONE, MD mario.campone@ico.unicancer.fr
Principal Investigator: Mario CAMPONE, MD
Institut Sainte-Catherine
Recruiting
Avignon, France
Contact: Alice Mege, MD a.mege@isc84.org
Principal Investigator: Alice Mege, MD
Polyclinique Bordeaux Nord Aquitaine
Recruiting
Bordeaux, France, 33077
Contact: Nadine DOHOLLOU, MD n.dohollou@bordeauxnord.com
Contact: Nadine DOHOLLOU, MD
Institut Bergonié
Recruiting
Bordeaux, France
Contact: Hervé Bonnefoi, MD h.bonnefoi@bordeaux.unicancer.fr
Principal Investigator: Hervé Bonnefoi, MD
Centre François Baclesse
Recruiting
Caen, France
Contact: Christelle Lévy, MD c.levy@baclesse.unicancer.fr
Principal Investigator: Christelle Lévy, MD
Centre Jean Perrin
Recruiting
Clermont-Ferrand, France
Contact: Marie-Ange Mouret Reynier, MD marie-ange.mouret-reynier@cjp.fr
Principal Investigator: Marie-Ange Mouret Reynier, MD
Ch Alpes Leman
Not yet recruiting
Contamine Sur Arve, France, 74130
Contact: Carol ALLIOT, MD calliot@ch-alpes-leman.fr
Contact: Carol ALLIOT, MD
Centre Georges François Leclerc
Recruiting
Dijon, France, 21079
Contact: Nicolas Isambert, MD nisambert@cgfl.fr
Principal Investigator: Nicolas Isambert, MD
Chd Vendee
Recruiting
La Roche-sur-Yon, France, 85925
Contact: Tifenn L'Haridon, MD tifenn.lharidon@chd-vendee.fr
Contact: Tifenn L'Haridon, MD
Centre Oscar Lambret
Recruiting
Lille, France
Contact: Nuria KOTECKI, MD
Principal Investigator: Nuria KOTECKI, MD
Chu Dupuytren
Not yet recruiting
Limoges, France, 87000
Contact: Laurence VENAT-BOUVET, MD laurence.venat-bouvet@chu-limoges.fr
Contact: Laurence VENAT-BOUVET, MD
Hopital Privé Jean Mermoz
Not yet recruiting
Lyon, France, 69008
Contact: Olfa DERBEL, MD o.derbelmermoz@gmail.com
Contact: Olfa DERBEL, MD
Centre Hospitalier Lyon Sud
Recruiting
Lyon, France
Contact: Benoit You, MD
Principal Investigator: Benoit You, MD
Centre Léon Bérard
Recruiting
Lyon, France
Contact: Thomas Bachelot, MD thomas.bachelot@lyon.unicancer.fr
Principal Investigator: Thomas Bachelot, MD
Institut Paoli Calmettes
Recruiting
Marseille, France
Contact: Anthony Gonçalves, MD goncalvesa@ipc.unicancer.fr
Principal Investigator: Anthony Gonçalves, MD
Institut Régional du Cancer Montpellier Val d'Aurelle
Recruiting
Montpellier, France
Contact: William Jacot, MD william.jacot@icm.unicancer.fr
Principal Investigator: William Jacot, MD
Centre Alexis Vautrin
Recruiting
Nancy, France
Contact: Elisabeth Luporsi, MD e.luporsi@nancy.unicancer.fr
Principal Investigator: Elisabeth Luporsi, MD
Institut de Cancérologie de l'Ouest/ René Gauducheau
Recruiting
Nantes, France
Contact: Mario Campone, MD mario.campone@ico.unicancer.fr
Principal Investigator: Mario Campone, MD
Centre Antoine Lacassagne
Recruiting
Nice, France
Contact: Jean-Marc Ferrero, MD jean-marc.ferrero@nice.unicancer.fr
Principal Investigator: Jean-Marc Ferrero, MD
Institut Curie
Recruiting
Paris, France
Contact: Marie-Paule Sablin, MD mariepaule.sablin@curie.fr
Principal Investigator: Marie-Paule Sablin, MD
Centre Eugène Marquis
Recruiting
Rennes, France
Contact: Claudia Lefeuvre-Plesse, MD c.lefeuvre@rennes.unicancer.fr
Principal Investigator: Claudia Lefeuvre-Plesse, MD
Centre Henri Becquerel
Recruiting
Rouen, France
Contact: Jean-Christophe Théry, MD
Principal Investigator: Jean-Christophe Théry, MD
Institut Curie
Recruiting
Saint-Cloud, France
Contact: Florence COUSSY, MD florence.coussy@curie.fr
Principal Investigator: Florence COUSSY, MD
Hopitaux Universitaire de Strasbourg - Hopital Civil
Recruiting
Strasbourg, France
Contact: Philippe BARTHELEMY, MD philippe.barthelemy@chru-strasbourg.fr
Principal Investigator: Philippe BARTHELEMY, MD
Hopitaux Du Leman
Recruiting
Thonon-les-Bains, France, 74200
Contact: Francesco DEL PIANO, MD f-delpiano@ch-hopitauxduleman.fr
Contact: Francesco DEL PIANO, MD
Institut Claudius Regaud
Recruiting
Toulouse, France
Contact: Florence Dalenc, MD dalenc.florence@iuct-oncopole.fr
Principal Investigator: Florence Dalenc, MD
Gustave Roussy
Recruiting
Villejuif, France
Contact: Monica Arnedos, MD Monica.ARNEDOS@gustaveroussy.fr
Principal Investigator: Monica Arnedos, MD
Sub-Investigator: Fabrice André, MD Collapse << |
| NCT02449655 | Advanced Gastric Adenocarcinom... more >>a Collapse << | Phase 2 | Recruiting | December 2019 | Korea, Republic of
... more >>
Samsung Medical center
Recruiting
Seoul, Korea, Republic of, 135-710
Contact: Yoonjeong Ahn 82-2-2148-7395 younjeong.ahn@samsung.com
Principal Investigator: Jeeyun Lee, MD,PhD Collapse << |
| NCT03182634 | Advanced Breast Cancer | Phase 2 | Recruiting | November 2023 | United Kingdom
... more >> Royal Marsden Hosital, Sutton
Recruiting
Surrey, England, United Kingdom, SM2 5PT
Royal Bournemouth Hospital
Recruiting
Bournemouth, United Kingdom
Addenbrooke's Hospital
Recruiting
Cambridge, United Kingdom
Royal Devon and Exeter Hospital
Recruiting
Exeter, United Kingdom
Beatson West of Scotland Cancer Centre
Recruiting
Glasgow, United Kingdom
Royal Marsden Hospital
Recruiting
London, United Kingdom
University College Hospital London
Recruiting
London, United Kingdom
Christie Hospital
Recruiting
Manchester, United Kingdom Collapse << |
| NCT02576444 | Cancer | Phase 2 | Recruiting | March 2019 | United States, Connecticut
... more >>
Yale Cancer Center
Recruiting
New Haven, Connecticut, United States, 06520-8028
Contact: Alexandra Minnella 203-737-3446 alexandra.minnella@yale.edu
Principal Investigator: Joseph P Eder, MD
United States, Massachusetts
Dana-Farber Cancer Institute
Recruiting
Boston, Massachusetts, United States, 02215
Contact: Brian Beardslee 617-632-5638 brian_beardslee@dfci.harvard.edu
Principal Investigator: Geoffrey Shapiro, MD
United States, Ohio
Cleveland Clinic Taussig Cancer Institute
Recruiting
Cleveland, Ohio, United States, 44195
Contact: Nancy Frazier 216-444-5531 frazien@ccf.org
Principal Investigator: Davendra PS Sohal, MD, MPH
United States, Tennessee
Vanderbilt-Ingram Cancer Center
Recruiting
Nashville, Tennessee, United States, 37232
Contact: Tom Leonard-Martin 615-936-6726 Thomas.Leonard-Martin@vumc.org
Principal Investigator: Vicki L Keedy, MD Collapse << |
| NCT01692262 | Metastatic Castrate-Resistant ... more >>Prostate Cancer (mCRPC),
Efficacy,
Safety and Tolerability,
Pharmacokinetics,
Pharmacodynamics,
Tumour Response. Collapse << | Phase 1 | Completed | - | United States, Florida
... more >>
Research Site
Sarasota, Florida, United States
United States, Massachusetts
Research Site
Boston, Massachusetts, United States
United States, Michigan
Research Site
Ann Arbor, Michigan, United States
United States, New Jersey
Research Site
Hackensack, New Jersey, United States
United States, Tennessee
Research Site
Nashville, Tennessee, United States
United Kingdom
Research Site
Cardiff, Wales, United Kingdom
Research Site
London, United Kingdom
Research Site
Southampton, United Kingdom Collapse << |
| NCT01992952 | Estrogen Receptor Positive Bre... more >>ast Cancer Collapse << | Phase 1
Phase 2 | Active, not recruiting | October 2020 | United Kingdom
... more >> Christie Hospital
Manchester, Greater Manchester, United Kingdom
Ysbyty Gwynedd
Bangor, United Kingdom
Clatterbridge Cancer Centre
Bebington, United Kingdom
Royal Blackburn Hospital
Blackburn, United Kingdom
Blackpool Victoria Hospital
Blackpool, United Kingdom
Velindre NHS Trust
Cardiff, United Kingdom
University Hospital of North Durham
Durham, United Kingdom
Great Western General Hospital
Edinburgh, United Kingdom
Calderdale and Huddersfield NHS Foundation Trust
Huddersfield, United Kingdom
The Ipswich Hospital NHS Trust
Ipswich, United Kingdom
University Hospitals Morecambe Bay
Lancaster, United Kingdom
St James's University Hospital
Leeds, United Kingdom
Mount Vernon Cancer Centre
London, United Kingdom
Royal Free Hospital
London, United Kingdom
Derriford Hospital
Plymouth, United Kingdom
Royal Preston Hospital
Preston, United Kingdom
Glan Clwyd Hospital
Rhyl, United Kingdom
Queen's Hospital
Romford, United Kingdom
Southampton General Hospital
Southampton, United Kingdom
Royal Stoke University Hospital
Stoke-on-Trent, United Kingdom
Royal Albert and Edward Infirmary -Wrightington
Wigan, United Kingdom Collapse << |
| NCT01226316 | Advanced Solid Malignancy
... more >> Safety and Tolerability
Pharmacokinetics
Pharmacodynamics
Tumour Response
Advanced or Metastatic Breast Cancer
Ovarian Cancer
Cervical Cancer
Endometrial Cancer
PIK3CA
AKT1
PTEN
ER Positive
HER2 Positive Collapse << | Phase 1 | Recruiting | November 15, 2019 | - |
| NCT02451956 | Advanced Gastric Cancer | Phase 2 | Recruiting | December 2018 | Korea, Republic of
... more >>
Samsung Medical Center
Recruiting
Seoul, seoul, korea, Republic of, Korea, Republic of, 135-710
Contact: yoon Jeong Ahn 221487395 ext 82
Principal Investigator: Seung Tae Kim, M.D., Ph.D. Collapse << |
| NCT01625286 | Advanced or Metastatic Breast ... more >>Cancer
ER+ve Advanced or Metastatic Breast Cancer Collapse << | Phase 1
Phase 2 | Active, not recruiting | March 29, 2019 | - |
| NCT01895946 | Advanced Solid Malignancy,
... more >> Safety and Tolerability,
Pharmacokinetics, Pharmacodynamics,
Tumour Response, Collapse << | Phase 1 | Completed | - | Netherlands
... more >> Research Site
Amsterdam, Netherlands, 1066 CX
United Kingdom
Research Site
Manchester, United Kingdom, M20 4BX
Research Site
Surrey, United Kingdom, SM2 5PT Collapse << |
| NCT01353781 | Advanced Solid Malignancy
... more >> Advanced Solid Tumor Collapse << | Phase 1 | Completed | - | Japan
... more >> Research Site
Chuo-ku, Japan Collapse << |
| NCT02423603 | Metastatic Breast Cancer | Phase 2 | Active, not recruiting | December 2018 | - |
| NCT02077569 | Invasive Breast Cancer | Phase 2 | Completed | - | United Kingdom
... more >> Royal Derby Hospital
Derby, Derbyshire, United Kingdom, DE22 3DT
Plymouth Hospitals NHS Trust
Derriford, Plymouth, Devon, United Kingdom, PL6 8DH
Royal Bournemouth Hospital
Bournemouth, Dorset, United Kingdom, BH7 7DW
Poole Hospital NHS Foundation Trust
Poole, Dorset, United Kingdom, BH15 2JB
Leicester Royal Infirmary
Leicester, Leicestershire, United Kingdom, LE1 5WW
Western General Hospital
Edinburgh, Lothian, United Kingdom, EH4 2XU
Royal Liverpool University Hospital
Liverpool, Merseyside, United Kingdom, L7 8XP
Kingsmill Hospital
Sutton-in-Ashfield, Nottinghamshire, United Kingdom, NG17 4JL
Sheffield Cancer Research Centre
Sheffield, South Yorkshire, United Kingdom, S10 2SJ
University Hospital Birmingahm
Birmingham, West Midlands, United Kingdom, B15 2TT
Leeds St James Institue of Oncology
Leeds, West Yorkshire, United Kingdom, LS9 7TF Collapse << |
| NCT02208375 | Breast Cancer
... more >> Malignant Female Reproductive System Neoplasm Collapse << | Phase 1
Phase 2 | Active, not recruiting | November 2021 | United States, Texas
... more >>
University of Texas MD Anderson Cancer Center
Houston, Texas, United States, 77030 Collapse << |
| NCT02525068 | Adenocarcinoma of the Prostate | Phase 2 | Recruiting | March 2020 | United Kingdom
... more >> Royal Marsden Hospital
Recruiting
Sutton, Surrey, United Kingdom, SM2 5PT
Principal Investigator: Johann De Bono, Professor Collapse << |
| NCT01895946 | - | - | Completed | - | - |
| NCT03772561 | Solid Tumor, Adult | Phase 1 | Recruiting | December 31, 2023 | Singapore
... more >> National University Hospital
Recruiting
Singapore, Singapore, 119074
Contact: David SP Tan 65 6779 5555 david_sp_tan@nuhs.edu.sg Collapse << |
| NCT02121639 | Prostate Cancer | Phase 1
Phase 2 | Recruiting | December 2018 | United Kingdom
... more >> Southampton General Hospital
Recruiting
Southampton, Hampshire, United Kingdom, SO16 6YD
Contact: Sara Moein S.Hosseini-Moein@soton.ac.uk
Principal Investigator: Simon Crabb Collapse << |
| NCT03310541 | Breast Cancer
... more >> Prostate Cancer
Advanced Solid Tumors Collapse << | Phase 1 | Recruiting | October 2020 | United States, New Jersey
... more >>
Memoral Sloan Kettering Monmouth
Recruiting
Middletown, New Jersey, United States, 07748
Contact: David Hyman, MD 646-888-4544
United States, New York
Memorial Sloan Kettering Westchester
Recruiting
Harrison, New York, United States, 10604
Contact: David Hyman, MD 646-888-4544
Memorial Sloan Kettering Cancer Center
Recruiting
New York, New York, United States, 10065
Contact: David Hyman, MD 646-888-4544
Contact: Alison Schram, MD 646-888-5388
Principal Investigator: David Hyman, MD Collapse << |
| NCT02338622 | Advanced Cancer | Phase 1 | Unknown | October 2015 | United Kingdom
... more >> Northern Institute for Cancer, Freeman Hospital
Recruiting
Newcastle, Newcastle upon Tyne, United Kingdom, NE7 7DN
Contact: Elizabeth R Plummer, MD +44(0)191 213844 ruth.plummer@newcastle.ac.uk
Principal Investigator: Elizabeth R Plummer, MD
Royal Marsden NHS Foundation Trust
Recruiting
Sutton, Surrey, United Kingdom, SM2 5PT
Contact: Timothy A Yap, PhD 0208 661 3539 timothy.yap@icr.ac.uk
Principal Investigator: Timothy A Yap, PhD Collapse << |
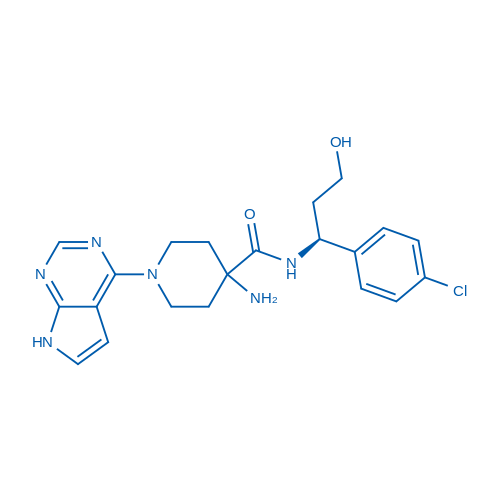
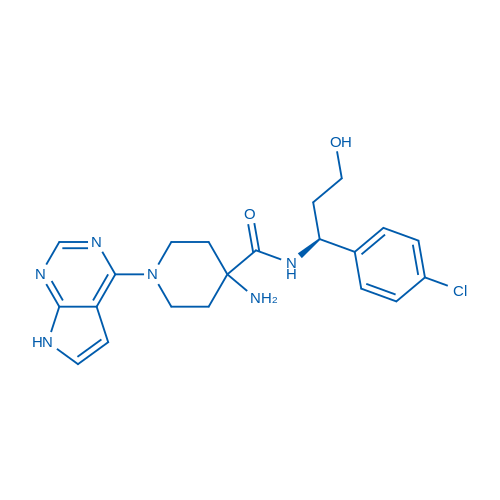

 400-920-2911
400-920-2911 sales@csnpharm.cn
sales@csnpharm.cn tech@csnpharm.cn
tech@csnpharm.cn
