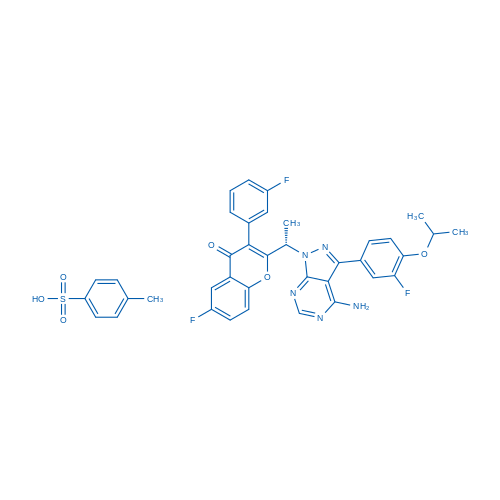
CAS No.: 1532533-72-4
Umbralisib tosylate Catalog No. CSN51112
Synonyms: TGR-1202 tosylate;RP5264 tosylate
Umbralisib tosylate is a novel phosphatidylinositol 3-kinase delta (PI3Kdelta) inhibitor under development at TG Therapeutics. In 2021, the product received accelerated approval in the U.S. for the oral, once-daily treatment of adult patients with relapsed or refractory marginal zone lymphoma (MZL) who have received at least one prior anti-CD20-based regimen and for the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) who have received at least three prior lines of systemic therapy. Launch took place short after. In 2021, TG Therapeutics completed a rolling submission in the U.S in combination with ublituximab for the treatment of chronic lymphocytic leukemia (CLL). The product is in phase III clinical trials, as monotherapy or in combination with ublituximab, for the treatment of CLL. The product is also in phase II/III clinical trials, as monotherapy or in combination with ublituximab, for the treatment of relapsed or refractory small lymphocytic lymphoma and B-cell lymphomas, including MZL, FL, diffuse large B-cell lymphoma and mantle cell lymphoma. The company refers to the combination regimen of ublituximab and TGR-1202 as TG-1303. The drug is also in phase II clinical development for the treatment Waldenstrom macroglobulinemia. Phase I clinical trials are ongoing for the treatment of Hodgkin's lymphoma. Phase I clinical trials were completed for the treatment of patients with select relapsed or refractory solid tumors, such as adenocarcinoma of the pancreas, adenocarcinoma of the colon, rectum, gastric and GE junction cancer, and GI Stromal Tumor (GIST). In 2016, orphan drug designation was assigned to the compound in the U.S. for the treatment of CLL. In 2017, additional orphan drug designation was granted in the U.S. for the treatment of CLL and DLBCL, in combination with ublituximab. In January 2019, the FDA granted breakthrough therapy designation to the product for the treatment of adult patients with MZL who have received at least one prior anti-CD20 regimen. The compound was granted additional orphan drug designation in the U.S. for the treatment of treatment of nodal, extranodal, and splenic MZL in 2019, and for the treatment of FL in 2020. Originated by Rhizen Pharmaceuticals, the product was jointly developed by Rhizen Pharmaceuticals and TG Therapeutics since 2012. In 2014, exclusive global development and commercialization rights (excluding India) were licensed to TG Therapeutics. In 2020, the product received fast track designation in US by TG Therapeutics for the treatment of adult patients with CLL in combination with ublituximab.
| 规格 | 价格 | 促销价格 | 库存 | 数量 |
|---|

靶点选择性
生物活性
实验方案
- 计算器
- 储存液制备
技术信息
| CAS号 | 1532533-72-4 | 储存条件 |
|
|
| 分子式 | C38H32F3N5O6S | 运输 | 蓝冰 | |
| 分子量 | 743.75 | 别名 | TGR-1202 tosylate;RP5264 tosylate |
 400-920-2911
400-920-2911 sales@csnpharm.cn
sales@csnpharm.cn tech@csnpharm.cn
tech@csnpharm.cn
