CAS No.: 1232416-25-9
贝索塞替尼 Catalog No. CSN15802
Synonyms: VX970;M6620;Berzosertib
VE-822 is an ATR inhibitor with IC50 of 19 nM in HT29 cells.
| 规格 | 价格 | 促销价格 | 库存 | 数量 |
|---|

纯度 & 质量文件
批次:
靶点选择性
生物活性
靶点 ATM
Ki:34nMATR
Ki:0.2nMDNA-PK
Ki:4μMmTOR
Ki:1μM- 描述
- 作用机制
- 细胞研究
- Cell Data
动物研究 剂量 Mice[1] (p.o.): 60 mg/kg
给药途径 p.o.
- 临床研究
NCT号 适应症或疾病 临床期 招募状态 预计完成时间 地点 NCT03641313 Clinical Stage III Gastric Can... more >>cer AJCC v8 Clinical Stage III Gastroesophageal Junction Adenocarcinoma AJCC v8 Clinical Stage IV Gastric Cancer AJCC v8 Clinical Stage IV Gastroesophageal Junction Adenocarcinoma AJCC v8 Clinical Stage IVA Gastric Cancer AJCC v8 Clinical Stage IVA Gastroesophageal Junction Adenocarcinoma AJCC v8 Clinical Stage IVB Gastric Cancer AJCC v8 Clinical Stage IVB Gastroesophageal Junction Adenocarcinoma AJCC v8 Metastatic Gastric Adenocarcinoma Metastatic Gastroesophageal Junction Adenocarcinoma Pathologic Stage III Gastric Cancer AJCC v8 Pathologic Stage III Gastroesophageal Junction Adenocarcinoma AJCC v8 Pathologic Stage IIIA Gastric Cancer AJCC v8 Pathologic Stage IIIA Gastroesophageal Junction Adenocarcinoma AJCC v8 Pathologic Stage IIIB Gastric Cancer AJCC v8 Pathologic Stage IIIB Gastroesophageal Junction Adenocarcinoma AJCC v8 Pathologic Stage IIIC Gastric Cancer AJCC v8 Pathologic Stage IV Gastric Cancer AJCC v8 Pathologic Stage IV Gastroesophageal Junction Adenocarcinoma AJCC v8 Pathologic Stage IVA Gastroesophageal Junction Adenocarcinoma AJCC v8 Pathologic Stage IVB Gastroesophageal Junction Adenocarcinoma AJCC v8 Postneoadjuvant Therapy Stage III Gastric Cancer AJCC v8 Postneoadjuvant Therapy Stage III Gastroesophageal Junction Adenocarcinoma AJCC v8 Postneoadjuvant Therapy Stage IIIA Gastroesophageal Junction Adenocarcinoma AJCC v8 Postneoadjuvant Therapy Stage IIIB Gastroesophageal Junction Adenocarcinoma AJCC v8 Postneoadjuvant Therapy Stage IV Gastric Cancer AJCC v8 Postneoadjuvant Therapy Stage IV Gastroesophageal Junction Adenocarcinoma AJCC v8 Postneoadjuvant Therapy Stage IVA Gastroesophageal Junction Adenocarcinoma AJCC v8 Postneoadjuvant Therapy Stage IVB Gastroesophageal Junction Adenocarcinoma AJCC v8 TP53 Gene Mutation Unresectable Gastroesophageal Junction Adenocarcinoma Collapse << Phase 2 Not yet recruiting May 31, 2020 - NCT02567422 Head and Neck Squamous Cell Ca... more >>rcinoma Human Papillomavirus Negative Stage III Nasal Cavity and Paranasal Sinus Squamous Cell Carcinoma AJCC v6 and v7 Stage III Oropharyngeal Squamous Cell Carcinoma AJCC v7 Stage IV Nasal Cavity and Paranasal Sinus Squamous Cell Carcinoma AJCC v7 Stage IV Oropharyngeal Squamous Cell Carcinoma AJCC v7 Stage IVA Nasal Cavity and Paranasal Sinus Squamous Cell Carcinoma AJCC v7 Stage IVA Oropharyngeal Squamous Cell Carcinoma AJCC v7 Stage IVB Nasal Cavity and Paranasal Sinus Squamous Cell Carcinoma AJCC v7 Stage IVB Oropharyngeal Squamous Cell Carcinoma AJCC v7 Stage IVC Nasal Cavity and Paranasal Sinus Squamous Cell Carcinoma AJCC v7 Stage IVC Oropharyngeal Squamous Cell Carcinoma AJCC v7 Collapse << Phase 1 Recruiting - United States, California ... more >> City of Hope Comprehensive Cancer Center Recruiting Duarte, California, United States, 91010 Contact: Site Public Contact 800-826-4673 becomingapatient@coh.org Principal Investigator: Erminia Massarelli University of California Davis Comprehensive Cancer Center Recruiting Sacramento, California, United States, 95817 Contact: Site Public Contact 916-734-3089 Principal Investigator: Jonathan W. Riess United States, Connecticut Smilow Cancer Center/Yale-New Haven Hospital Recruiting New Haven, Connecticut, United States, 06510 Contact: Site Public Contact 203-785-5702 canceranswers@yale.edu Principal Investigator: Barbara A. Burtness Yale University Recruiting New Haven, Connecticut, United States, 06520 Contact: Site Public Contact 203-785-5702 canceranswers@yale.edu Principal Investigator: Barbara A. Burtness United States, Georgia Emory University Hospital Midtown Recruiting Atlanta, Georgia, United States, 30308 Contact: Site Public Contact 888-946-7447 Principal Investigator: Conor E. Steuer Emory University Hospital/Winship Cancer Institute Recruiting Atlanta, Georgia, United States, 30322 Contact: Site Public Contact 404-778-1868 Principal Investigator: Conor E. Steuer United States, Kentucky University of Kentucky/Markey Cancer Center Recruiting Lexington, Kentucky, United States, 40536 Contact: Site Public Contact 859-257-3379 Principal Investigator: Susanne M. Arnold United States, Maryland Johns Hopkins University/Sidney Kimmel Cancer Center Suspended Baltimore, Maryland, United States, 21287 United States, Ohio Case Western Reserve University Suspended Cleveland, Ohio, United States, 44106 Ohio State University Comprehensive Cancer Center Recruiting Columbus, Ohio, United States, 43210 Contact: Site Public Contact 800-293-5066 Jamesline@osumc.edu Principal Investigator: Darrion L. Mitchell United States, Virginia University of Virginia Cancer Center Recruiting Charlottesville, Virginia, United States, 22908 Contact: Site Public Contact 434-243-6303 PAS9E@virginia.edu Principal Investigator: Varinder Kaur United States, Wisconsin University of Wisconsin Hospital and Clinics Recruiting Madison, Wisconsin, United States, 53792 Contact: Site Public Contact 800-622-8922 Principal Investigator: Justine Yang-Bruce Collapse << NCT02595892 Ovarian Serous Tumor ... more >> Recurrent Fallopian Tube Carcinoma Recurrent Ovarian Carcinoma Recurrent Primary Peritoneal Carcinoma Collapse << Phase 2 Recruiting - United States, Arizona ... more >> Mayo Clinic Hospital Recruiting Phoenix, Arizona, United States, 85054 Contact: Site Public Contact 855-776-0015 Principal Investigator: Andrea E. Wahner Hendrickson Mayo Clinic in Arizona Recruiting Scottsdale, Arizona, United States, 85259 Contact: Site Public Contact 855-776-0015 Principal Investigator: Andrea E. Wahner Hendrickson United States, California UC San Diego Moores Cancer Center Recruiting La Jolla, California, United States, 92093 Contact: Site Public Contact 858-822-5354 cancercto@ucsd.edu Principal Investigator: Michael T. McHale University of California San Diego Recruiting San Diego, California, United States, 92103 Contact: Site Public Contact 858-822-5354 cancercto@ucsd.edu Principal Investigator: Michael T. McHale United States, Florida Mayo Clinic in Florida Recruiting Jacksonville, Florida, United States, 32224-9980 Contact: Site Public Contact 855-776-0015 Principal Investigator: Andrea E. Wahner Hendrickson United States, Massachusetts Massachusetts General Hospital Cancer Center Recruiting Boston, Massachusetts, United States, 02114 Contact: Site Public Contact 877-726-5130 Principal Investigator: Panagiotis A. Konstantinopoulos Brigham and Women's Hospital Recruiting Boston, Massachusetts, United States, 02115 Contact: Site Public Contact 888-823-5923 ctsucontact@westat.com Principal Investigator: Panagiotis A. Konstantinopoulos Beth Israel Deaconess Medical Center Recruiting Boston, Massachusetts, United States, 02215 Contact: Site Public Contact 617-667-9925 Principal Investigator: Panagiotis A. Konstantinopoulos Dana-Farber Cancer Institute Recruiting Boston, Massachusetts, United States, 02215 Contact: Site Public Contact 877-442-3324 Principal Investigator: Panagiotis A. Konstantinopoulos United States, Michigan Wayne State University/Karmanos Cancer Institute Recruiting Detroit, Michigan, United States, 48201 Contact: Site Public Contact 313-576-9790 ctoadmin@karmanos.org Principal Investigator: Ira S. Winer United States, Minnesota Mayo Clinic Recruiting Rochester, Minnesota, United States, 55905 Contact: Site Public Contact 855-776-0015 Principal Investigator: Andrea E. Wahner Hendrickson United States, Pennsylvania Thomas Jefferson University Hospital Recruiting Philadelphia, Pennsylvania, United States, 19107 Contact: Site Public Contact 215-955-6084 Principal Investigator: Russell J. Schilder University of Pittsburgh Cancer Institute (UPCI) Recruiting Pittsburgh, Pennsylvania, United States, 15232 Contact: Site Public Contact 412-647-8073 Principal Investigator: Alexander B. Olawaiye United States, Tennessee Vanderbilt University/Ingram Cancer Center Recruiting Nashville, Tennessee, United States, 37232 Contact: Site Public Contact 800-811-8480 Principal Investigator: Marta A. Crispens United States, Texas M D Anderson Cancer Center Recruiting Houston, Texas, United States, 77030 Contact: Site Public Contact 877-312-3961 Principal Investigator: Siqing Fu United States, Virginia University of Virginia Cancer Center Recruiting Charlottesville, Virginia, United States, 22908 Contact: Site Public Contact 434-243-6303 PAS9E@virginia.edu Principal Investigator: Linda R. Duska United States, Wisconsin University of Wisconsin Hospital and Clinics Recruiting Madison, Wisconsin, United States, 53792 Contact: Site Public Contact 800-622-8922 Principal Investigator: Lisa M. Barroilhet Collapse << - 更多
- 参考文献
- [1] Fokas E, Prevo R, et al. Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumors to radiation. Cell Death Dis. 2012 Dec 6;3:e441.
- [2] Josse R, Martin SE, et al. ATR inhibitors VE-821 and VX-970 sensitize cancer cells to topoisomerase i inhibitors by disabling DNA replication initiation and fork elongation responses. Cancer Res. 2014 Dec 1;74(23):6968-79.
实验方案
- 计算器
- 储存液制备
技术信息
| CAS号 | 1232416-25-9 | 储存条件 |
|
|||||||||||||
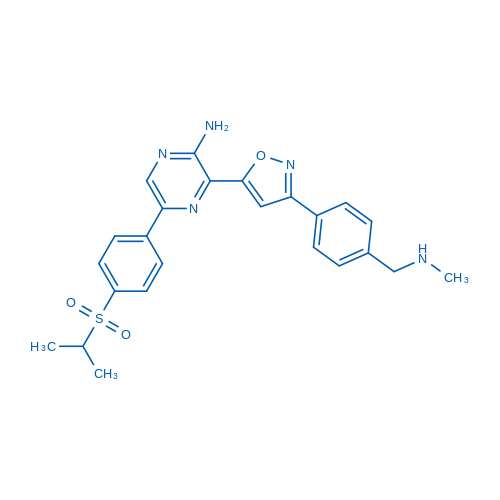
| 分子式 | C24H25N5O3S | 运输 | 蓝冰 | |||||||||||||
| 分子量 | 463.55 | 别名 | VX970;M6620;Berzosertib | |||||||||||||
| 溶解度 |
|
动物实验配方 |
|
 400-920-2911
400-920-2911 sales@csnpharm.cn
sales@csnpharm.cn tech@csnpharm.cn
tech@csnpharm.cn
